
True reflectionThe power of an artificial stem cell
Barry Behr, PhD, preps for surgery with deep, calming breaths. Each incision that follows has to be precise enough to slice an object a tenth the size of the period at the end of this sentence. There are no do-overs. Using a laser, he tediously grazes an embryo, causing the shell to crack and spill its goopy contents into the liquid milieu of the petri dish.
Photograph by Barry Behr
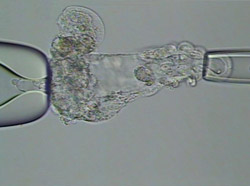
Barry Behr uses a laser to crack an embryo and release the inner cell mass — the source of embryonic stem cells. The cell mass, not yet emerged, is at the embryo’s left side. Magnification: 500x.
“This is called ‘assisted hatching,’” Behr says as he glances up from the laser-equipped microscope in the center of his spotless in vitro fertilization lab.
He normally performs this micro-operation only on embryos he’s preparing to implant into a hopeful mother-to-be. But last year, he used it for an experiment comparing embryonic stem cells with artificial ones — called induced pluripotent stem cells, or iPS cells. He and his colleagues wanted to know if iPS cells could replace embryonic stem cells — a gold standard for studying disease.
Behr collected stem cells from an embryo carrying a mutation for Marfan syndrome — a condition that affects connective tissue, causing extra-long limbs, tall height and cardiovascular problems. His collaborators used ordinary skin cells from an adult Marfan patient to engineer iPS cells.
“We had both types of stem cells side by side, both containing the defective gene responsible for Marfan. This was a perfect opportunity to compare them,” says surgeon Michael Longaker, MD, senior author of the study, which was published Jan. 3 in Proceedings of the National Academy of Sciences.
They found the iPS cells perfectly mirrored Behr’s embryonic cells, establishing an easier, less controversial alternative for life-saving research. But collecting the embryonic stem cells wasn’t easy.
An IVF patient with Marfan syndrome donated the week-old embryo after Behr “spell checked” its genetic code and found that it harbored an error in the gene Fibrillin-1, the root of Marfan symptoms. In addition to an assisted hatch, Behr used the laser to cull the dense, stem-cell-containing inner mass — similar to the yolk of an egg — from the rest of the embryo. The maneuver carries only a 20 to 30 percent success rate. And Behr had only one embryo, one shot.
“This is where spatial memory comes in handy,” Behr says. He places an embryo-containing petri dish on the microscope’s platform for a demonstration viewed on its monitor. To keep the microscopic ball of cells from spinning in its liquid buffer, Behr uses two tiny, joystick-controlled glass straws that apply gentle suction from both sides. Once he sucks the embryo in place, he uses his mouse to aim the laser at the stem-cell-harboring mass that looks like a wad of gum stuck to the inside of a bubble.
With the straws steady, Behr shoots with consecutive clicks of a foot-pedal trigger, cutting through the embryo one laser blast at a time. If it spins or floats away, he has to remember the orientation of the wad. It’s a painstaking process, to say the least. Once the clump is completely severed, his collaborators plunk it into stem cell nutrient broth for months to ensure he sliced out only the stem cells. It took a year to authenticate the Marfan embryonic stem cell line.
“It certainly would be a lot easier to have an alternative cell line without this slicing and dicing,” Behr says. What’s so cool about the group’s study — cooler than a laser-firing microscope, he says — is that they proved they had one.
— Beth Mole


