
Plus
The case of the disappearing liver disease
Uncovering an ordinary antibiotic’s secret power
By Erin Digitale
Portrait by Leslie Williamson
The 15-year-old boy’s lab tests indicated his liver function was badly impaired. He had a double whammy of two serious gastrointestinal diseases, both lacking cures. On top of it all, his colon was infected with an aggressive bacterial strain, Clostridium difficile.
Although pediatric gastroenterologist Kenneth Cox, MD, had little to offer for the teen’s other problems, he could at least treat the infection. He prescribed the antibiotic vancomycin.
And something very strange happened. The liver-disease symptoms vanished.
“At first I thought it was a coincidence,” says Cox, now chief medical officer at Lucile Packard Children’s Hospital, recalling the moment in 1993 when he saw the first hint of improvement. Maybe he had misattributed symptoms of infection to liver disease, he thought. “But then I stopped the antibiotic, and the liver disease came back, even though the infection was gone.”
So Cox, who is also associate chair of pediatrics and senior associate dean for pediatric and obstetric clinical affairs at the Stanford School of Medicine, gave a second round of vancomycin. Once again, the teenager’s appetite returned, his pain disappeared and his liver tests normalized.
Cox tried vancomycin in a handful of other patients who shared the teen’s liver and colon diagnoses but had never had C. difficile. These kids had been told that their liver disease, primary sclerosing cholangitis, was untreatable. Even a liver transplant was not a guaranteed cure — the disease could recur and destroy a new organ. Yet with vancomycin, the PSC disappeared.
The discovery left Cox in an unusual position. A coincidence — a serendipitous colon infection, of all things — left him holding a potential silver bullet for a devastating and poorly understood pathology.
“The problem is that I’m dealing with a very small group of kids with an unusual disease,” he says. “How do I get the science to prove that vancomycin works, so that all of my colleagues would say, ‘This is the therapy’?”
Unexplained destruction
PSC starts in the “biliary tree,” the tree-shaped network of tubes that carry newly manufactured bile from the liver through the bile duct to the intestine, where bile aids digestion and absorption of dietary fat. In PSC, for reasons no one understands, the tubes become blocked by inflammation. So bile backs up, destroying liver cells and eventually causing cirrhosis.
The rare disease, which occurs in about 10 people of every million, leaves patients feeling severely unwell, with abdominal pain, itching, jaundice, poor appetite, deep fatigue and signs of malnourishment. It can hit people of any age. About three-quarters of PSC patients — including the 15-year-old who started Cox’s research odyssey — also have the more-common diagnosis of inflammatory bowel disease, another poorly understood condition, which is characterized by inflammation and ulceration of the intestine, diarrhea, abdominal pain and a host of other problems.
Cox and his Stanford collaborators believe that if they can figure out how vancomycin alleviates PSC, they’ll solve two mysteries at once. Not only will they have the evidence to convince other physicians that vancomycin is a good PSC treatment, but by finding out how the drug works, they may also learn how PSC begins — which may open doors to better therapies.
Although the research task is daunting, beneath Cox’s caution about its challenges is a definite sense of excitement.
“Most discoveries come by careful observation. I feel lucky that I’ve made this observation,” he says. “The remarkable part is, not only do the liver tests get better, but the children also feel so much better. If you take a look at these children before and after therapy, they don’t look like the same child.”
Rescuing a toddler’s liver
One of the most dramatic vancomycin-induced transformations came in 2005, after Cox’s team suggested the drug to Lyn Woodward and Melissa Hartman. Their little girl had been through the diagnostic wringer.
Things began to go wrong for Ellery Woodward-Hartman at 8 months of age, when her growth started to lag behind that of her twin brother, Robert. Her liver function gradually worsened; no one could figure out why. By the time she turned 2, Ellery’s liver was scarred with cirrhosis and she was badly jaundiced. Before Cox saw her, other physicians had tested Ellery for everything from cystic fibrosis to lymphoma to HIV. None of those diagnoses fit, and her liver was getting worse. Woodward and Hartman were told to anticipate a liver transplant.
“I thought, this can’t be happening,” Hartman says.
In late October 2005, Cox’s pediatric gastroenterology fellow, Anca Safta, MD, read Ellery’s chart. The symptoms lined up with PSC, Safta and Cox agreed. Safta proposed vancomycin treatment to the family.
“She said, ‘I know about Ellery; we have something that can help her,’” Woodward says, recalling her first conversation with Safta.
“There’s a line from Emily Dickinson: ‘Hope is the thing with feathers,’” Hartman says. “I thought of that poem. It was such a relief.”
‘Most discoveries come by careful observation. I feel lucky that I’ve made this observation.’
Cox wasn’t sure a child as sick as Ellery would benefit from the therapy; his other patients had mostly been at earlier stages of PSC illness. Maybe the antibiotic would at least give her a few months to get stronger before a transplant, he told Woodward and Hartman. Cox wrote the prescription and told the family he would follow up with them soon.
Clues from the clinic
Since 1993, Cox has tried vancomycin on every PSC patient he’s treated, slowly accumulating evidence for the drug’s effects. In 2008, he published clinical observations of the first 14 patients, showing the drug caused improvement in blood markers of liver failure. The index patient, now an adult, is no longer in Cox’s care, but last Cox knew, he continued to do well. To date, 33 of Cox’s patients, plus a handful of others cared for by colleagues around the country, have received the drug. But it’s still largely unknown as a PSC therapy.
Funding has been one obstacle to advancing the research. So far, the work has proceeded without traditional funding sources such as NIH grants. Instead, patients’ families have financed the research via a parent-launched nonprofit, the Children’s PSC Foundation. Cox is now working to secure pharmaceutical company funding for a multicenter study to enable researchers to try the drug in a larger group of adults and children.
LifeART MediClip image, Wolters Kluwer
ealth Inc.- Lippincott Williams & Wilkins
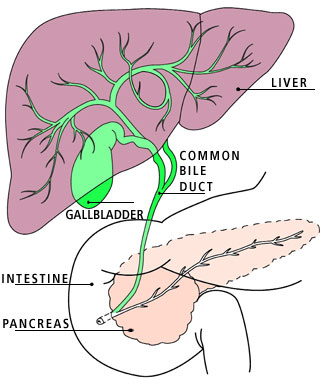
Bile normally moves from the liver through the branching network of ducts to the intestine, where it digests dietary fat. In primary sclerosing cholangitis, a rare liver disease, inflammation blocks the ducts. As a result, bile backs up, damaging the liver.
Filling the knowledge gaps
In spite of the limited resources, Cox has assembled a multidisciplinary team of Stanford collaborators to figure out how vancomycin works. The scientists are starting from one important clue: They know oral vancomycin, the drug formulation Cox uses for PSC, is not absorbed from the intestine. Yet PSC’s trail of destruction starts with inflammation outside the intestine, in the tubes that drain bile from the liver to the gut. The drug must be acting at a distance — but how?
One hypothesis is that PSC arises when pathogenic bacteria in the gut backflow into the bile duct and start a destructive inflammatory response. Normally, everything moves down the duct in one direction, from liver to intestine.
“Essentially, this would be regurgitation of bacteria into the bile drainage system,” says project collaborator David Relman, MD, a professor of infectious diseases and of microbiology and immunology at Stanford.
Another possibility is that bacteria somehow escape from the gut to the blood, then travel through the blood to the bile duct and trigger inflammation.
Under these hypotheses, which Relman’s laboratory is starting to investigate, vancomycin would resolve PSC with its antibiotic action, killing gut bacteria. To determine if that’s happening, the researchers are first taking a census of the bacterial communities in healthy children’s small intestines.
“Almost everything we know so far about the usual gut microbe community is based on adults,” Relman says.
The researchers plan to compare gut microbes in healthy kids to those in PSC patients before and after vancomycin. Their major obstacle — indeed, the reason we know so little about kids’ gut microbes — is the difficulty of sampling the small intestine’s contents. It would be unethical to perform invasive endoscopy on children who have no medical indication for the procedure, so the control samples in Relman’s new study will come from kids receiving endoscopy to investigate non-PSC complaints such as chronic abdominal pain. It’s also challenging to find “control” children who have not received recent courses of antibiotics. “That we know messes with the normal picture,” Relman says.
Still, he is optimistic about the lab’s prospects for cataloguing the gut microbes of kids with and without PSC. If kids with PSC have “different” bacteria before vancomycin treatment and return to a normal bacterial profile with the drug, it would provide strong circumstantial evidence that bacteria initiate PSC. And it would be a good starting point for studies of how the bacteria incite disease.
An unexpected modus operandi
Another possibility, however, is that in PSC vancomycin is acting as more than an antibiotic. Though textbooks label it a bacteria-killer, the Stanford team suspects vancomycin is also changing patients’ inflammatory response.
Although the idea might seem strange at first, there’s a well-established precedent for antibiotics quieting inflammation. In the last decade, several groups of researchers have demonstrated that, for example, tetracycline’s anti-inflammatory activity contributes to its effectiveness against rheumatoid arthritis, that macrolide antibiotics reduce inflammation in chronic airway disease, and that amoxicillin lowers bowel inflammation in ulcerative colitis.
If vancomycin is acting as an anti-inflammatory in PSC, says Kari Nadeau, MD, PhD, an assistant professor of pediatric immunology and allergy at Stanford, that suggests PSC is a disease of immune function run amok.
Scott Seki of Nadeau’s group already has some enlightening preliminary data. Regulatory T cells, the immune cells that prevent autoimmune disease by tamping down the inflammatory response, exhibit interesting changes during vancomycin treatment, he has found.
Using blood samples drawn before and after vancomycin therapy, Seki showed that the drug doubles PSC patients’ levels of regulatory T cells. Evidence from other autoimmune diseases suggests this change is big enough to cause therapeutically useful drops in inflammation — in other words, it may explain why vancomycin works. Two other experiments in Nadeau’s lab also pointed to regulatory T cells as key players in the vancomycin response. However, this information is still drawn from observations of a very small group of patients, so the team is now working to expand and strengthen their data.
If the findings about the regulatory T cells do turn out to be the PSC linchpin, Nadeau says, “We might infer that some kind of inflammatory process is turned on early in the life of these children that we should move quickly to try to regulate.”
And if the antibacterial effects of vancomycin are key, Relman says, the best approach would be to design a drug that gets rid of PSC-provoking bacteria but acts more selectively. Right now, vancomycin is probably killing beneficial bacteria that have nothing to do with PSC, he adds. “We’d rather not be using a sledgehammer if something more precise and elegant could be devised.”
Surprise ending
In mid-November 2005, Ellery Woodward-Hartman’s case was presented to the transplant selection committee at Packard Children’s. Though medical records from her pre-vancomycin days clearly pointed toward transplant, the liver-function tests performed after her first 10 days on vancomycin looked promising. The committee decided to re-evaluate her case in December.
A few weeks later, after about a month on vancomycin, Ellery and her family saw their physicians again. “Dr. Safta and Dr. Cox couldn’t believe how well she looked,” Woodward recalls. Ellery’s jaundice had cleared up. Her belly, previously swollen with fluids that accumulated when her liver function was at its worst, had returned to a healthy shape. She was still tiny in comparison with her twin but she was more energetic.
And by the time the transplant committee reconsidered her case, it was clear that the vancomycin was a success. Ellery didn’t need a liver transplant.
“With the degree of disease she had, I was very surprised,” says Safta, now an assistant professor of pediatric gastroenterology at the University of Maryland. Woodward and Hartman feel extremely grateful for the compassionate care Safta provided when Ellery was at her worst, and they still send periodic updates. “It’s just amazing where Ellery has gotten to,” Safta says. “She’s probably the only one with such severity of cirrhosis that has turned the corner like this.”
Now, after more than five years on vancomycin, Ellery is a thriving 7-year-old. Like other patients taking the drug for PSC, she continues to use it without side effects. Though her liver still bears the scars of cirrhosis, and there’s a possibility she may need a transplant at a future date, her liver-function tests are now normal. Her growth has caught up to her brother’s.
“I can’t even calculate what Dr. Cox has been able to do for Ellery,” Woodward says.
Cox sees Ellery’s case as a gratifying success, and he’s encouraged that emerging Stanford science supports the therapy he discovered by accident. This type of discovery is “one of the rewards of being an academic physician,” he says. His collaborators agree. In a project like this, “the patients talk to you through their data,” says Nadeau. “If they’re getting better, that’s what you take as real. That’s what inspires you to go back to the lab and figure out what is happening.”
But there’s one last hurdle: Cox worries that too many patients like Ellery are never offered vancomycin. More than 6,000 people have received liver transplants because of PSC, he says. Though it sounds like a large number, the disease is rare enough that many gastroenterologists never see a case — and so they aren’t reading the literature about new treatment advances. To try to bridge the gap, Cox has partnered with the Children’s PSC Foundation in hopes of helping physicians and patients’ families learn about the treatment.
At a recent foundation fundraiser, he got to meet a few children who had received the therapy from other doctors.
“Kids came up to say it had changed their lives,” Cox says. “They were so thankful. That makes me think this is the right thing to be doing.”


