SUMMER 2008 CONTENTS
Home
Good as Gold?
New drug approvals ebb; doubts over testing's gold standard grow
Q&A with Katie Couric
Standing up for cancer research
Breath of Hope
Lifeline or gamble? Sometimes a clinical trial is both
Just Another Lab Rat
The human subjects trade is booming, largely without oversight
Fixing Trial Tribulations
Solutions from Stanford
A Spoonful of Sugar Pills
Why nothing really is something, and in some ways is better than anything
Banding Together
Minds of all kinds join to hasten discoveries of new medical treatments

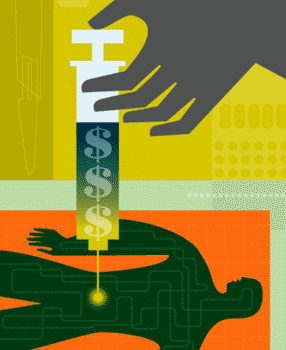
Just Another
Lab Rat
The human subjects trade is booming, largely without oversight
By Tracie White
Illustration by Paul Wearing
Paul Clough is a rat. Over the past four years, the perfectly healthy 29-year-old former day laborer has spent 500 nights living in research centers, given blood 1,200 times and swallowed more than 700 pills. By participating in clinical trials and repeatedly donating his body to science, he’s earned $90,000.
The skin in the crook of his right arm is thick with red scarring from the thousand-odd blood tests. Once a nurse nicked a nerve while drawing blood and he couldn’t use his arm for a full year. He has routinely suffered the common side effects of diarrhea, headaches and body rashes. He’s lived off bland food and shared a room full of bunk beds for months at a time with total strangers. He’s been told when and where to urinate, when and what to eat, to avoid exercise. It all goes with the territory, he says. Clough and his fellow lab rats, as he calls research subjects, have been the first to test a variety of potential new medications for everything from high cholesterol to tuberculosis to AIDS to fungus.
“My longest clinical study was a heart drug trial for 30 days,” Clough says. “I made about $5,000 for that one.”
For him, it’s a good way to make a living. He still lives paycheck to paycheck, but he’s paying off old gambling debts and the job has its perks.
“It’s much better than flipping hamburgers,” Clough says. “And instead of helping just one person, you’re helping millions.”
“In the old days, when I first started in trials, people did it more for the sake of doing it, you know, for society. But now it’s just all about people making the quickest buck. People tend to forget there’s a higher level to the whole thing.”
Clough is part of a growing subculture of professional “human guinea piggers” who have emerged over the past 10 to 20 years, making a living off the explosion in the multibillion-dollar for-profit clinical trials industry. Students, the unemployed, ex-cons, young people living on the margins — estimated millions of healthy people long on free time and short on cash have discovered the increasing amounts of money to be made testing medical products.
No one knows for sure how many people are involved because no one keeps track of the actual numbers, but it is known that pharmaceutical companies spend $40 billion on clinical trials each year. Adil Shamoo, PhD, a bioethicist at the University of Maryland School of Medicine and co-founder of Citizens for Responsible Care and Research, estimates (based on National Institutes of Health data on expenditures per subject and available statistics on private investment in research) that in the United States about 25 million people now participate in research each year. That’s up from his estimate of 18 million in 1997.
The emergence of this new population has many critics worried. They see it as the ultimate outgrowth of a new for-profit world of clinical trials, one that is spinning out of control. Lacking sufficient oversight, it’s putting participants at risk and raising doubts about the validity of drug trials.
Unlike the majority of human subjects who join trials in the hopes of finding a treatment for a medical problem, healthy subjects expect no therapeutic benefit to balance the risks they take. And as much as researchers hope that people participate in trials for altruistic reasons, the reality is, most healthy subjects do it for the money.
The question then becomes, how much money is too much?
What is certain is that there’s more money to be made participating in clinical trials today than ever before.
Most of the professional guinea piggers, as they call themselves, follow the particularly high-paying, phase-1 drug trials — the first experimentation in humans — around the country, receiving up to $10,000 per trial. If you play your cards right, drug-trial veterans say, you can pretty easily make $20,000 to $30,000 a year. You’re not going to get rich, but if you’re unemployed, a student, or, say, trying to pay off gambling debts, it can look pretty good.
Clough certainly admits he takes part in trials for the money, but that’s not the only reason. He first heard about clinical trials four years ago while working as a day laborer in Kansas City when “some homeless dude” told him he could earn $600 for a three-night clinical study. All he had to do was give blood, take some pills and survive a little boredom.
“I liked it right off,” Clough says. Within a year, he’d moved to Austin — one of the meccas for clinical trials in the United States — and has been living off research studies ever since. Clough had a tough family life, bounced around in the foster-care system, lived in residential homes and spent some time behind bars for minor infractions. Which is, in part, why clinical trials work for him.
“I like the institutional setting,” says Clough. “I like things that are well-structured. I haven’t had a lot of structure in my life. When I’m not in a study, my life is in disarray.”
But, he says, there’s more to it than that. He likes the idea that he could be helping to bring a new AIDS drug to market, for example, or slow the rate of heart disease.
“In the old days, when I first started in trials, people did it more for the sake of doing it, you know, for society. But now it’s just all about people making the quickest buck. People tend to forget there’s a higher level to the whole thing.”
Trials move off campus
Paying healthy human subjects to participate in clinical trials is nothing new. Scientists have been paying people to participate in trials for the past 100 years, going back to the days when Walter Reed Army Medical Center paid out $100 in gold to anyone who would participate in a yellow fever trial, says Christine Grady, PhD, a nurse with a doctorate in bioethics who heads the section on human subjects research at the National Institutes of Health. Not only does she believe it’s ethical to pay people, it might be unethical not to pay them, she says.
But what is new is the changing scene of human experimentation.
Once the purview of academia where oversight systems have been in place for decades, clinical trials have increasingly become a business.
Pharmaceutical and biotech companies have entered the scene, bringing with them the need for more subjects and faster studies in the race to bring more products to market.
In 1991, 80 percent of industry-sponsored trials were conducted in an academic setting, according to The New England Journal of Medicine. But over the past couple of decades, as the pharmaceutical industry has grown increasingly impatient with the slow pace of academia, it’s turned to the private sector, hiring third-party, for-profit testing centers called contract research organizations, or CROs, to run their trials.
Today more than 70 percent of clinical trials are conducted in the private sector.
Giant, new, for-profit trial centers have popped up all around the country: Boston, Philadelphia, Chicago, Indianapolis, San Diego, Miami to Roanoke, Va. And a growing percentage, upward of about 50 percent, of clinical trials now take place overseas.
“The relationship between testers and test subjects has become, more nakedly than ever, a business transaction,” says Carl Elliott, MD, PhD, professor of bioethics at the University of Minnesota. Elliott, who recently wrote about the world of guinea piggers for The New Yorker, first stumbled across the term when he read a copy of Guinea Pig Zero: A Journal for Human Research Subjects, published by a legend in the world of guinea pigging, Bob Helms.
Helms, now a union organizer for hospital workers in Philadelphia, worked as a professional guinea pigger for eight years until he turned 46 in 2003 and became too old to qualify for most trials. He started publishing Guinea Pig Zero, an online zine, to provide a meeting place for fellow guinea piggers, where they could share stories, compare notes and advocate for fair treatment. Filled with black humor, the journal went so far as to give letter grades to various research centers, prompting at least one lawsuit against Helms. Instead of “research participants,” Guinea Pig Zero writers called themselves “brain sluts,” or “medical meat puppets,” or today’s more commonly used “lab rats.”
“In its own small way, Guinea Pig Zero was revolutionary,” Elliott writes in the literary journal Tin House. “Before Guinea Pig Zero, nobody had really ever thought about research subjects as a kind of community. Nobody had thought of guinea pigging as a job, or that guinea pigs might band together and agitate for better pay and better conditions. It also offered a window into a world that most of us never see, even those of us who work in hospitals and medical schools.”
Sure lab rats do this for the money, Helms says. You’d be crazy to do it and not get paid. He’s seen friends “zonked out” following psychiatric drug trials, he’s swallowed a radioactive isotope sandwich for money, he’s followed every disaster story of deaths and disability and suicide related to trials gone awry that make it into the press.
Any time you participate in a clinical trial, you’re taking a risk. It’s the nature of the beast. That’s what you get paid for. Guinea piggers are doing a job; they deserve reasonable pay and good working conditions, Helms says. Still, he appreciates the ethical concern of trying to determine just how much researchers and drug companies should pay their human subjects. And the need for an oversight system that works.
He says certain lines just shouldn’t be crossed.
“There’s the urban legend of the guy who had his big toe cut off and sewn back on with laser beams for $10,000,” says Helms. “And the guy who swears he was in a study that paid him $100,000 to stop his heart and start it back up again.”
He laughs. Sure, people know these stories aren’t true. But you offer enough money for just about anything, and people will line up.
How much is too much?
No set guidelines determine how much to pay human participants in clinical trials, says the NIH’s Grady, who recently conducted a study on how institutions set payment levels for human subjects. Federal guidelines maintain payments not be “coercive,” but they don’t say exactly how to do this.
“It’s pretty arbitrary,” she says. “We interviewed a variety of institutions, pharmaceutical companies, academic centers — everybody said they had some rules of thumb, less than a third had any written rules. The take-home message was that the majority did not clearly spell out how they came out with the amount that they would pay. There were even variations within the same institutions.”
Much of the ethics oversight for clinical trials thus rests on the institutional review boards, or IRBs — panels of experts set up to review every U.S. trial. IRBs were originally established in the 1970s in the wake of various medical research scandals like the U.S. Public Health Service’s Tuskegee syphilis study. That study, begun in 1932, left black men with syphilis untreated for 40 years until the story was uncovered in 1972.
Stanford’s IRB panels determine rates of pay on a case-by-case basis depending primarily on the time and effort required of participants. The IRB’s written guidelines state: “The level of payment should not be so high as to cause a prospective participant to accept risks that he or she would not accept in the absence of payment.”
“We try to determine what is a fair-market value,” says Sean Mackey, MD, PhD, a Stanford associate professor of anesthesiology, explaining how he determines how much to pay healthy volunteers in his on-campus clinical trials. “We need to price these studies such that it pays for people’s time but doesn’t blind them to the potential risks.” Stanford’s IRB is key to this decision-making process, Mackey says.
“The IRB keeps an eye on how much money people are being paid and it wants to know every detail.”
In the past, when academic centers were the primary location for clinical trials, the IRBs were primarily nonprofit volunteer groups, like Stanford’s, located on academic campuses made up of volunteer experts, mostly faculty.
Today, there are about 10,000 IRBs that review the ethics of studies in exchange for a fee. If a company doesn’t like what one IRB tells it, it can shop around until it finds another one that tells it what it wants to hear, says bioethicist Shamoo. IRBs themselves have become part of the for-profit world of clinical trials, he says.
“You and I and your cousin could form an IRB,” Shamoo says. “It’s highly decentralized.”
Troubles begin to bubble
In May 2006, the largest drug-testing site in North America — 675 beds — was ordered demolished by Florida’s Miami-Dade County because of fire and safety violations. According to numerous media reports, SFBC International (the company has since changed its name to PharmaNet Development Group) was paying undocumented immigrants to participate in clinical trials in an overcrowded, poorly maintained former Holiday Inn. Last August, SFBC International paid $28.5 million to settle a class-action lawsuit.
“The FDA is looking at modernizing its regulations to better reflect the emergence of all these different parties now participating in clinical trials with human subjects to make sure they all are fully accountable.”
In 2003, a previously healthy college student named Traci Johnson committed suicide in Eli Lilly labs after being paid to take a new version of an antidepressant.
In 1996, The Wall Street Journal reported that Eli Lilly was using homeless alcoholics from a local shelter to test experimental drugs at its testing site in Indianapolis. Two years earlier, the Food and Drug Administration issued a rebuke to Lilly for using alcoholics in a drug study that triggered the company’s most public drug-testing disaster, resulting in the deaths of five healthy subjects due to toxic liver reactions. A series of scandals like these involving healthy clinical trial subjects have made headlines over the past decade or so, prompting calls for changes in the oversight system.
“There’s been a whole litany of problems,” says David Magnus, PhD, director of the Stanford Center for Biomedical Ethics, who points out that sufficient oversight is essential in preventing both unethical and accidental tragedies from occurring in the clinical trial industry.
“This is why IRBs have a really important role to play,” says Magnus. And why he’s concerned about the emergence of the for-profit IRB system. For-profit IRBs pose a greater potential for conflict of interests and less rigorous oversight, he says.
The Food and Drug Administration acknowledges apparent oversight problems in this new for-profit world of clinical trials. During the past few years, the FDA has held a series of public meetings and issued at least 11 guidance documents related to clinical trials addressing these concerns.
“The FDA is looking at modernizing its regulations to better reflect the emergence of all these different parties now participating in clinical trials with human subjects to make sure they all are fully accountable,” says Rachel Behrman, MD, a deputy director with the agency. The agency recommends using a centralized IRB review process and advocates improved monitoring of reports of adverse events, but no new regulations have yet been implemented.
Many human-rights advocates like Shamoo insist piecemeal changes such as these simply aren’t enough. Shamoo argues that the increasing amounts of money and trials are obviously coercing the poor, the homeless, the disenfranchised in our society into participating in trials.
Though data describing the demographics of phase-1, healthy research subjects are lacking, it stands to reason that the overwhelming majority are poor, uneducated minorities and the politically powerless, says Shamoo.
“If you just go one day and look who goes to these trials, especially in urban areas, it’s the mentally ill and drug addicts lining up outside.”
For years, Shamoo has pushed for legislation in the form of the National Human Subject Protection Act, which would at least provide as much protection for humans as animals already receive. He says the legislation has some support in Congress, but he expects no action during the current administration.
“One hundred percent of all research with animals has been governed and regulated since 1966 by the Animal Welfare Act,” Shamoo says. “That’s not true for humans. It’s a broken, unfair system that everyone’s trying to repair at the edges. You really need a whole new law.”
The lack of oversight is also putting all who take medications at risk, Shamoo says. Many trial participants fear being dropped from studies if they report side effects, and the major oversight agency, the FDA, is understaffed, so adverse affects are greatly underreported, he says. “You only hear of the nightmare cases when they get in the press.”
Last year, a report by the inspector general of the Department of Health and Human Services found that the FDA inspected just 1 percent of study sites. “Small wonder, since it has a mere 200 investigators and there are 350,000 sites,” writes Shamoo and a co-author in the March-April 2008 Hastings Center Report.
“Given the lack of oversight, it is not a surprise that adverse events are underreported,” they write.
The view from inside
The nightmare stories don’t worry Clough. If you’re careful and follow the rules, the risks of taking untried and potentially dangerous drugs remain relatively low, he insists. He encourages his fellow lab rats to follow all rules, to report all adverse reactions.
It’s important “not to be stupid,” he says.
Always obey “wash-out” periods — 30-day breaks between trials — to purge any residual drugs; never participate in more than one trial at a time. Don’t lie. And save your money for the gaps between trials.
During the endlessly boring hours spent hanging out in clinics with nothing much to do except fight over the TV remote control, Clough has gone online and created his own Web site, JustAnotherLabRat.org (jalr.org). The site lists locations of trials and offers tips on how to be a successful lab rat. He’s trying to pick up where Bob Helms left off, Clough says, by providing an online community for human guinea piggers.
Among Clough’s key tips: Always obey “wash-out” periods — 30-day breaks between trials — to purge any residual drugs; never participate in more than one trial at a time, since that can prove dangerous, even deadly. Don’t lie. And save your money for the gaps between trials. Don’t gamble it all away.
“A lot of people cheat,” Clough says. “They do more than one study in a 30-day period. They check out of one and check into another the next day. Some people report false adverse events. They know the ones that will get them kicked out early so that they can leave and still get their money.”
Sure, homeless people participate in the trials, he says. He’s known homeless people who use the money to get back on their feet, “druggies” who use the time to dry out, ex-cons with few options who enter straight from prison. But if you follow the rules, anyone can be a good guinea pigger and make a living out of it.
Clough has participated in a total of 26 clinical trials: He crisscrosses the country, stopping in Kansas City, New Jersey, Wisconsin, Indiana and Texas. He intends to continue working as a human guinea pig until he turns 45 and, like Helms, gets too old to qualify for most studies.
In the meantime, he’ll continue giving the best advice he can to the average newcomer: “You will either love it or hate it. You’ll be excited at the prospect of making lots of money in a short amount of time. You’ll be terrified of the blood draws and the idea of taking an experimental drug. You may as well give it a try.”
For Clough, it’s one way of escaping the rat race.
