The brain’s silent majority
How the other 90 percent of your brain works
By Bruce Goldman
Illustration by Joe Morse
When you have no clue, call it glue.
“Glia,” the Greek word for glue, was the name the pathologist Rudolph Virchow gave, back in 1856, to the gelatinous substance that forms the bulk of the brain. And it stuck. These days, scientists use it to denote the matter that accounts for 90 percent of the brain’s cells and more than half its volume — but, like the late comic Rodney Dangerfield, “can’t get no respect.” Neurons, the “talented tenth” of the human brain that hog the lion’s share of brain scientists’ attention, are indeed a work of evolutionary art. They’ve got a knack that glia lack: Their aptitude for high-speed, long-distance communication makes them the nervous system’s premier information processors. Neurons can send electrical impulses whizzing down their long, tubular projections called axons, which hook up to other neurons via junctions called synapses. A single neuron may interconnect with as many as tens of thousands of others, forging circuits of a complexity that leaves computer scientists gasping. A huge part of neurobiology is focused on trying to figure out the significance of all those circuits, whose output can be monitored much as one taps a phone conversation: by plugging electrodes into the circuitry, and listening in. Glial cells’ activities are subtler. Unlike their flashier electronic cousins, glia speak in chemical whispers. Learning their language has been tougher. As a result, glial cells were long seen as inert nerve cement: just so many packing peanuts whose raison d’être is to keep our neurons from jiggling when we jog.
“When the brain is injured, the neighborhood astrocytes go into a completely altered state.”
We now know they’re doing much more. Perhaps most spectacular is the new evidence that one of the three major glial-cell types, known as astrocytes, have a lot to do with where synapses snap into place, which of them flourish and which die. Because the placement and relative strengths of the brain’s 100-trillion-plus synapses are so intimately associated with what defines us — learning, thinking, feeling, remembering and forgetting — in a strictly material sense one could say the soul resides in the synapses. So these heretofore-slighted astrocytes turn out to be a very big deal.
Certainly, it’s no stretch to imagine that knowing what glial cells do, and how they do it, could help explain brain disorders and how to cure them. But getting neuron-centric brain scientists to accept this is another matter entirely.
From time to time, Ben Barres brings a brain in to his office. “We neurobiologists want to understand how it develops, how it functions in life, how it contracts disease, what it is that makes us human,” says Barres, MD, PhD, professor and chair of neurobiology.
Barres says, “When you think about cells in the brain, pretty much the first thing you think about is neurons.” But it was glial cells that riveted Barres’ attention. “During my training as a neurologist, back in the 1980s, I looked at a lot of disease-affected brains under the microscope,” he says. “And every time I looked, I could see that the glia were altered. That got me curious.” He’s been working on them ever since, in the process becoming one of the world’s leading authorities on the cells and something of an evangelist for their worthiness for study.
The guardian angels of the synapses
Probably the least understood glial cells, astrocytes are the most common, constituting about half of all human brain cells. These cells are so named because abundant long, thin projections give them a starry look. Recently, Barres and others have pinpointed several of these cells’ star qualities.
Most neurobiologists are too polite to tell you that neurons, although talented, are slobs. And they’ve known for a couple of decades that astrocytes clean up after them. When an electrical signal jolts a synaptic junction, chemicals known as neurotransmitters are released. These molecules diffuse across the tiny synaptic gap and stimulate receptors on the neuron on the far side, variously triggering or inhibiting a similar burst in this “downstream” neuron. Astrocytes take care of housekeeping functions such as feeding the neurons (supplying nutrients, energy-rich molecules and neurotransmitter precursors) or mopping up after them (for example, speedily slurping up spent neurotransmitter molecules from the synapses so that the next signal will be a clean one).
Arne Hurty 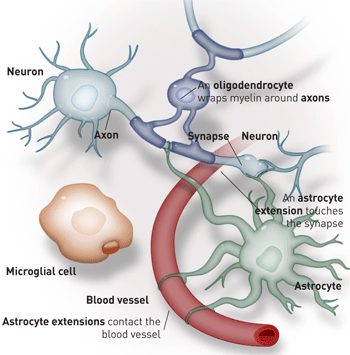 The other brain cells
The other brain cells
Neurons are the brain’s rock stars. But without the glial cells — astrocytes, microglia and oligodendrocytes — there would be no show at all.
But Barres had a hunch they did more. “The textbooks will tell you they mop up excess neurotransmitters there,” he says. “And it’s true. But we wondered, is that all?”
And now the lab has generated convincing evidence that astrocytes play a major role in controlling where and when our brain’s all-important synapses will form.
In that study, published in 2005, Barres and colleagues cultured highly purified mouse neurons in the total absence of glial cells. On the surface, at least, the neurons seemed fine. “They made axons, they even fired electrical signals. Everything looked good. Except for one thing — they were making hardly any synapses. And those that were produced didn’t work very well.”
Adding astrocytes, or solutions in which astrocytes had hung out (which presumably carried chemicals they secrete), reversed the mouse neurons’ overall synaptic deficiency. Barres and his team have since shown that adding a single protein secreted by astrocytes — it’s called thrombospondin — to the medium in which these purified neurons were growing boosted their synapse construction immensely.
Barres looked further into the role of this astrocyte protein, whose role in the brain had never been studied before. He found that astrocytes produce the protein during brain development, “at a time and place synapses are sprouting all over,” says Barres. Then, when brain maturation is complete, thrombospondin expression shuts down everywhere in the brain — except in the hippocampus, the part of the brain where new memories are formed, and one of the few places in the brain where constant, large-scale synapse formation is known to take place. “But when the brain is injured, the neighborhood astrocytes go into a completely altered state. They take on totally new properties. One of those is that they turn back on thrombospondin expression.” Barres asks: Could those astrocytes be playing a part in inducing and repairing synapses in the injured brain?
And then there’s this intriguing observation: Thrombospondin is one of only two genes that are far more highly expressed in human brains than in those of other primates.
The brains behind the operation
Nailing down astrocytes’ importance in creating new synapses was possible only because of the new lab techniques created by scientists, Barres prominent among them.
To best study how all the brain’s different cell types work together as a system, you first have to be able to tease them apart. Doing that was no mean feat, but Barres persisted. Since his graduate school days in neurobiology, Barres — aided, since joining Stanford’s faculty in 1993, by numerous postdocs and grad students — has figured out how to separate the brain into its cellular building blocks. “We can now purify each of these major classes of cells, put them into culture dishes and see what neurons do all by themselves and what they need glial cells for.”
That purification advance also allows for gene profiling. Virtually all of an organism’s cells have the same genes, but in one cell type only some genes work, while in a second type a different set are functional. Likewise, a healthy cell may turn on one set of genes, a sick cell another set. By extracting genetic material from purified cultures of single cell types and pouring the material over a “gene chip” — a device that can quickly quantify the extent to which genes are turned on within cells — Barres and his colleagues have learned which genes are active in each cell type, and at what levels.
Gene-profiling comparisons show that the three major glial-cell types are as different from one another as each is from a neuron, Barres says. That is, the genes these cell types turn on vary drastically. This has allowed the Barres group to harvest extremely specific markers for major brain-cell types, including markers revealing astrocytes that didn’t show up with commonly used previous labeling techniques.
“We can label a brain section and see where all of the astrocytes are, not just some of them. And they’re all over,” says Barres. This has led to new thinking. Imaging using these markers shows that astrocytes envelop neurons’ synapses. One such astrocyte can envelop thousands at a time.
Glial ordeals: A role in brain disease?
A key feature in healthy, young brains — the very ones in which it’s so critical for synapses to form — is, ironically, synapse death. That’s because the developing brain generates far more synapses than it needs. “The ‘good’ synapses stay, and some ‘bad’ extra synapses are pruned away,” says Barres, whose lab has found evidence that astrocytes play an active role there, too. In a study published in 2007, Barres and his colleagues showed that astrocytes cause some synapses to be covered with a protein called C1q. They had already known that whenever C1q coats the surface of bacterial or human cells on the far side of the blood-brain barrier, it marks them for destruction by the body’s immune system. And it appears to be marking synapses for destruction, too.
“If you’re gonna keep one cell alive in the stroke treatment, focus on the astrocytes!”
C1q-mediated synapse loss may turn out to be an important feature of such neurodegenerative disorders as Alzheimer’s, Lou Gehrig’s disease and glaucoma. The cardinal feature of all neurodegenerative disease is synapse loss, so drugs that block the C1q pathway may be useful.
“Expression of this protein shuts off in the adult brain,” says Barres. “But in neurodegenerative disease, it turns back on. Alzheimer’s disease is characterized by massive synapse loss. By the time of even the earliest detection of cognitive loss, as many as 80 percent of synapses may have already disappeared from some brain regions.”
Barres believes opportunities for putting this insight to practical use abound. “There’ve been a thousand failed clinical trials for stroke, all of them focused on keeping the neurons alive. But we know that the astrocytes make the chemical signals that keep the neurons alive. I keep hammering on this. If you’re gonna keep one cell alive in the stroke treatment, focus on the astrocytes!”
Rona Giffard, MD, PhD, professor of anesthesia, agrees. She is working with an animal model in which blood flow to the brain is briefly cut off, then reinstated, reliably destroying only a small set of neurons in a particularly vulnerable region of the brain. Giffard thinks the temporary oxygen deficit induced by the shutdown doesn’t immediately destroy the neurons themselves — “they take a week to die,” she notes — but rather impairs a key neuron-critical function of local astrocytes. In an experiment using rats, Giffard found that a drug that protects this faltering astrocyte function greatly increased the vulnerable neurons’ resistance to injury.
The fact that astrocytes in one small brain area seem so much more vulnerable than those in others suggests that astrocytes may differ from region to region. Giffard’s lab had previously identified differences in the response of astrocytes from different brain regions. Research in the Barres lab confirms that astrocytes are every bit as diverse as neurons are.
Joining the glia club
“There’s a loud sucking sound in this field as it draws in neuroscientists who, like neurons themselves, find themselves increasingly enmeshed with glial cells,” says Bruce Ransom, MD, PhD, who heads the University of Washington’s neurobiology department.
In 1986, Ransom, then on Stanford’s faculty, got the idea of starting a journal called Glia. At the time, the field was considered almost disreputable — “like parapsychology,” he says. The first issue came out in 1988. “That year, we barely managed to publish 300 pages. Now we could publish 4,000 pages a year if we wanted to, but we’re holding the line at about 2,000.”
Besides astrocytes, two other glial-cell types have important, if not exotic, functions in the brain. Oligodendrocytes, which account for 40 percent of the cells in the human brain, extrude a flagship fatty product, myelin, which insulates neuronal surfaces and speeds signals along their wire-like axons. Myelin is largely responsible for giving heavily myelinated regions of the brain (the “white matter”) their lighter color.
Another glial-cell type, microglia, constituting as much as 10 percent of the brain’s cells, serve an immune function in our brains, which are somewhat impervious to immune cells attempting entry from across the blood-brain barrier. Actually of immune origin, microglia are thought to migrate into the brain early in development, before the barrier is in place.
Every day, it’s a safer bet that glial cells are more than “nerve cement.” As one ascends the scale of evolutionary complexity, an increasing proportion of the brain’s cells are glial. In the simple nematode worm, they’re sparse; in a fruit fly, they’re up to 25 percent; in a mouse, about 65 percent. In a human brain, behind every great neuron stand nine great glial cells. “Doesn’t that tell us something?” Giffard asks.
Before we get too haughty, though, it should be noted that size undoubtedly has something to do with it. In an elephant, it’s 97 percent — “so, basically, glia with a few neurons thrown in,” says Barres.
And, hey, an elephant never forgets.
Bruce Goldman is at

Web Extra
Glia in action
Spying on glia
Astrocytes are known to send out numerous projections that contact both neurons and the tiny blood vessels that pervade the brain. This would put astrocytes in the catbird seat if one of their objectives were to alter blood flow in response to neuronal activity.
Earlier this year, three Stanford researchers uncovered evidence that that’s exactly what happens.
Axel Nimmerjahn, PhD, is a post-doctoral scholar in the laboratory of Mark Schnitzer, PhD, an assistant professor of biology and of applied physics. Together with then-graduate student Eran Mukamel, they used time-lapse microscopy to show, for the first time, that in physically active test animals, calcium signals, or flares, propagate from astrocyte to astrocyte over relatively huge distances in the animals’ cerebellum (a brain region associated with physical movement). Nearby blood vessels enlarged almost immediately after the flares. When the animals were awake but at rest, neither the flares nor subsequent blood-vessel dilation occurred. This supports the notion that astrocytes monitor neurons’ electrical activity levels and respond by generating calcium flares that, by yet unknown means, lead local blood vessels to supply more nutrients to the active site.
Microglia, too, have surprising jobs. In 2005, Nimmerjahn and two researchers at two separate Max Planck Institutes in Germany found that even in their “resting” state, microglia are amazingly active. Nimmerjahn and his co-investigators, using live-imaging time-lapse micros- copy, saw that microglia have long, slender projections extending to the surfaces of neurons, blood vessels and astrocytes. While the microglia themselves appeared stationary, their extensions were constantly probing first one, then another portion of other cells’ surfaces, as if monitoring their well-being. The team estimated that these microglial probes made a full sweep of the brain every few hours or so. When the team damaged a capillary with a laser beam, fingerlike extensions from numerous microglia soon converged on the scene and appeared to slurp up detritus.