Gut busters
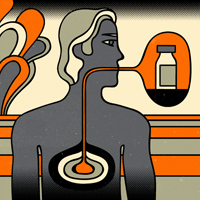
A regular dose of “good” bacteria not only improves gastric-bypass patients’ digestion — it helps them lose weight.
A study published this summer by John Morton, MD, associate professor of surgery, showed that patients who take probiotics after the most common stomach-shrinking surgery, the Roux-en-Y procedure, tend to shed more pounds than those who don’t take the supplements. Probiotics are the so-called good bacteria found in yogurt as well as in over-the-counter dietary supplements.
Illustration by Greg Mably
Morton, who wrote the paper with lead author Gavitt Woodard, a third-year medical student, and five other medical students, has performed more than 1,000 of these bypasses at Stanford Hospital & Clinics.
Morton says he was prompted to do the study because some patients have problems eating after gastric-bypass surgery, experiencing abdominal pain or bloating. “For some reason, the food doesn’t go down right,” he says.
When no anatomical reasons could be found for blockages, he hypothesized that a build-up of “bad” bacteria in the intestine might be the culprit, and that probiotics might be a solution.
The researchers followed 44 patients on whom Morton had performed the procedure from 2006 to 2007. Patients were randomized into either a probiotic or a control group. Both groups received the same surgical medical care and nutritional counseling, as well as the support of weight-loss study groups. Both groups also were allowed to consume yogurt, a natural source of probiotics. In addition, the probiotic group consumed one pill per day of Puritan’s Pride probiotic supplement, which is available online and in many stores. Morton has no financial ties to the company that produces it.
The study, published in the July Journal of Gastrointestinal Surgery, showed that at three months the probiotics group registered a 47.6 percent weight loss, compared with a 38.5 percent weight loss for the control group.
The study also found that levels of vitamin B-12 were higher in the patients taking probiotics — an important finding because patients often are deficient in B-12 after gastric-bypass surgery. The probiotics group had B-12 levels of 1,214 picograms per milliliter at three months, compared with the control group’s levels of 811 pg/mL.
“Part of the obesity puzzle may be due to the kind of bacteria you have in your intestine,” says Morton. He says he now recommends probiotic supplements to his patients. — Diane Rogers
This study was supported with no outside funding.
Ovary tests flunk
Current diagnostic tests for ovarian cancer are woefully ineffective for early detection of the disease, say Stanford researchers. A new study finds that ovarian cancer tumors usually turn deadly when they’re still far too small to unmask with current tests. To make a significant dent in the mortality rate for the deadly cancer, a test would have to detect tumors of less than 1 cm in diameter. None of today’s tests comes close to that level of performance.
Still, if that hurdle can be overcome, there is good reason to believe that testing could make a big difference. The window of opportunity for treating these clinically undetectable cancers before they become life-threatening is surprisingly long: about four years.
“We are miles away from detecting the most deadly ovarian tumors at this early stage,” says biochemistry professor Patrick Brown, MD, PhD, “but now we have a chance of actually designing an effective test that will allow us to treat them before they become deadly.” If a blood test is to be effective, says Brown, it will likely require identifying new markers that are never produced by normal cells — rather than testing for abnormally high levels of proteins detectable in normal blood, as current tests do. Other possible strategies might rely on new molecular imaging methods or fluid samples from the uterus or vagina — in which tumor markers are likely to be more concentrated.
The research was published in the July 28 issue of PLoS-Medicine. Ovarian cancer is a particularly scary disease because it is so difficult to detect before it spreads to surrounding organs and tissues.
“We are miles away from detecting the most deadly ovarian tumors at this early stage, but now we have a chance of actually designing an effective test that will allow us to treat them before they become deadly.”
“Reliable early detection would save so many more lives than many new blockbuster anticancer drugs,” says Brown, a Howard Hughes Medical Institute investigator, who collaborated on the study with the nonprofit Canary Foundation. “If we can do this, which is no small challenge, the potential to go from a less than 20 percent chance of surviving five years to a relatively minor surgery that would have a very high cure rate is huge,” he says.
Brown and his co-author, the Canary Foundation’s Chana Palmer, PhD, realized they could get a sneak peek at the tiny, most deadly ovarian tumors, known as serous tumors, by looking at data from studies of ovary tissue from women whose ovaries were removed preventively. Many women carrying the BRCA-1 cancer gene elect to have their ovaries removed, as the mutation increases their risk of developing ovarian cancer as well as breast cancer.
The researchers combined the results of several previously published studies of the ovarian tumors to estimate their prevalence, location, size and stage. By comparing this information with the incidence of diagnosed serous ovarian tumors in a similar group of women, they calculated the window of opportunity for early detection and possible successful treatment: about 4.3 years. During most of this time, the tumors were less than 1 cm in diameter. — Krista Conger
The Canary Foundation and Howard Hughes Medical Institute funded this study.
National stimulus
Sean Mackey, MD, PhD, has been studying the roots of pain in the brain, applying last year for a grant from the National Institutes of Health to continue his imaging studies in the field. But the grant was held up by a shortage of cash at the federal agency.
Now, thanks to the national economic stimulus program, Mackey, an associate professor of anesthesia, will receive $318,000 to expand his work, in which he uses real-time brain imaging to help individuals guide their brain activities. The research will not only help in pain management but may also shed light on addiction, cognitive development, depression and brain injury, he says.
And the funds will enable him to hire two full-time employees, in addition to providing faculty salary support for him and his collaborators in radiology, psychiatry and psychology, he says.
“It’s a wonderful opportunity,” Mackey says. “We expect the resources from this grant will ultimately benefit a number of groups beyond our lab. There’s much interest in real-time fMRI across the campus.”
Mackey is among at least 75 researchers at the School of Medicine who have been helped by the national stimulus package, which was signed into law in February and includes $10 billion for the NIH — $8.2 billion of which is for projects conducted outside the agency.
As of Sept. 24, the total of the grants to the medical school through the stimulus program was more than $50 million for projects on a wide range of topics, including studies on eye disease, cancer, tissue engineering and transplantation.
“Until it was announced that the stimulus program would include research funding, our medical school faculty and their colleagues across the country were increasingly distraught that scientific advances were being undermined by the downturn in NIH funding that began in 2003,” says the medical school’s dean, Philip Pizzo, MD. “Great ideas that missed the bar for the ever-decreasing, peer-reviewed funding priorities from the NIH meant that faculty were laying off staff, shrinking their efforts and shelving promising opportunities to bring new knowledge to improve the health of our nation.”
Pizzo notes that regardless of how economic pressures lead to health-care reform, the biggest savings will come from new discoveries and advances that will lead to more effective ways to treat and prevent disease. — Ruthann Richter
Transplant central
Ila Chakravarthy was 6 months old but weighed just 10 pounds. Then, in October 2008, she began vomiting up big clots of blood, and the condition of her failing liver grew worse. The time had come, her doctors said. Though her size and fragile health would make surgery perilous, Ila urgently needed a liver transplant.
Ila and her worried parents were referred to Lucile Packard Children’s Hospital, home of the nation’s busiest pediatric liver transplant team. The hospital’s team performed more liver transplants in 2008 — the most recent reporting period — than any other U.S. children’s hospital, according to the United Network for Organ Sharing. They also performed the largest number of surgeries in infants like Ila, who were younger than 1 year when they received a transplant. The team’s success rate, measured by patient and graft survival, suggested that if anyone could save Ila it was this team.
Greg Mably
“We’re known for taking care of very small children, transplanting much sicker patients and treating more kids with cancer than any other institution in the country,” says surgeon Carlos Esquivel, MD, PhD, chief of the division of transplantation.
Caring for the 45 children (including 18 infants) who received new livers at Packard last year required not just Esquivel’s surgical acumen, but the expertise of dozens of medical professionals: nurses, hepatologists, nephrologists, gastroenterologists, anesthesiologists, physician assistants and social workers. The team prepares patients for surgery and provides years of crucial follow-up care. They offer innovative treatment options developed at Stanford, such as kid-friendly immunosuppressive drugs, and operate the first clinic that helps teens take responsibility for their own care — a response to studies showing graft rejection rises in the teen years, perhaps because patients rebel by skipping doses of their immunosuppressive medication.
“When you’re doing a lot of transplants, especially difficult ones, you can get really successful at it and learn how to use your resources very effectively,” says pediatric hepatologist William Berquist, MD. For patients who receive transplants as babies and are followed at Packard through years of childhood and adolescence, he says, “we become like parents.”
The road to transplant
Ila’s liver began failing because of a congenital defect called biliary atresia — she lacked the tube connecting her liver and small intestine. Doctors told her parents, Maya Nanjundaswamy and Srinivas Chakravarthy, that Packard Children’s had the expertise to perform a difficult transplant in a small infant like their daughter.
The tiniest and sickest children, like Ila, need extra care at all stages of the transplant process. To make sure a child can handle surgery, the team’s medical subspecialists concentrate on preparatory measures such as preventing infection, treating bleeding and maximizing nutrition. In the operating room, babies require extra surgical skill: The hepatic artery in an infant may be only 1 to 2 millimeters in diameter, for example.
After a successful liver transplant, young patients thrive, making up for the slowed growth they experienced during liver failure.
The outlook for Ila’s future is excellent: She’s now an inquisitive toddler who grins toothily at her parents and is beginning to walk and talk. Says Esquivel, “These children are very resilient.” — Erin Digitale
Cheap genes
The first few times that scientists mapped out all the DNA in a human being, in 2001, each effort cost hundreds of millions of dollars and involved more than 250 people. Even last year, when the lowest reported cost was $250,000, genome sequencing still required almost 200 people.
Greg Mably
But this summer, professor of bioengineering Stephen Quake, PhD, sequenced his entire genome for less than $50,000 and with a team of just two other people over the course of one month. It’s one of fewer than a dozen sequenced worldwide.
“This is the first demonstration that you don’t need a genome center to sequence a human genome,” says Quake. “It’s really democratizing the fruits of the genome revolution and saying that anybody can play in this game.” He reported the accomplishment in the Aug. 9 online edition of Nature Biotechnology.
As sequencing becomes easier, more examples of the whole human genetic code are likely to be available for study, increasing understanding of how specific genes and mutations affect us. As costs drop, doctors could sequence their patients’ genomes and provide “personalized medicine” informed by the patient’s genetic profile.
Simpler sequencing
To sequence his genome, Quake’s team used a commercially available, refrigerator-sized instrument called the Helicos Biosciences SMS Heliscope. Quake, who pioneered the underlying technology in 2003, is a co-founder of the Cambridge, Mass.-based company and chairs its scientific advisory board.
“This is the first demonstration that you don’t need a genome center to sequence a human genome. It’s really democratizing the fruits of the genome revolution and saying that anybody can play in this game.”
The technology — the SMS in the instrument’s name — is called single molecule sequencing. While many techniques require generating thousands of copies of a subject’s DNA, the single molecule technique does not, reducing the cost and effort involved. Instead, the technique requires chopping the 3 billion or so fundamental units of DNA, called bases, into millions of strands about 30 bases long.
Each base of DNA (there are four: adenine, cytosine, guanine and thymine) links up with a specific other base. Quake’s technique takes advantage of these linking proclivities. The machine captures each of the millions of strands on a specially treated glass plate, holds them there and then washes successive batches of fluorescently labeled bases over the plate. For example, when adenine molecules wash over the plate, they attach to the thymine molecules in the attached DNA segments. In the end, an image of where these and the other bases bind reveals the sequence of each strand.
Computers assemble the strands back into a cohesive genome through comparison to the reference genomes that have been compiled before. The process is akin to assembling a huge jigsaw puzzle by referring frequently to the picture on the box.
Personal revelations
Quake’s genome has already yielded interesting facts. One is that he carries a rare mutation associated with a heart disorder. The good news, he says, is that he’s also apparently genetically predisposed to respond well to common cholesterol-lowering drugs.
On a lighter note, Quake’s code contains a form of a gene that has sometimes been associated with increased disagreeability.
“Of course, you don’t need my genome to tell you that,” Quake acknowledges. “My wife could have told you that and certainly the dean could have as well.”
— David Orenstein
Funding for the research came from the National Science Foundation and from the National Institutes of Health.
50 at Stanford
Hosting a gala celebration didn’t seem right, given the straightened finances of the medical school this year. So the school is recognizing the 50th anniversary of its move from San Francisco to Palo Alto in a serious fashion: More than 30 of the medical faculty, including the dean, are teaming up to teach a yearlong “mini med school” for the general public — a three-course introduction to human biology, health and disease, offered by Stanford Continuing Studies.
Stanford Medical History Center In 1957, construction was under way on campus for the medical center, which moved to Palo Alto from San Francisco 50 years ago.
In 1957, construction was under way on campus for the medical center, which moved to Palo Alto from San Francisco 50 years ago.
The decision to make the move from San Francisco wasn’t terribly popular at the time: Most of the San Francisco-based faculty chose not to move south, and many physicians in Palo Alto saw the new school as a threat. But the school’s leaders saw great potential in placing the medical school on the main Stanford campus, where the medical faculty and students could easily collaborate with other scientists and engineers.
“The decision was made and the future of Stanford Medicine was defined — with the result that 50 years later, the School of Medicine is one of the leading research-intensive medical schools in the world, a destiny that almost surely could not have been accomplished if the conventional wisdom of the time to leave the school in San Francisco had been followed,” wrote Philip Pizzo, MD, dean of the medical school, in his newsletter this summer.
Even without the gala, the anniversary has not gone unrecognized. Members of the 1959 faculty as well as other emeritus faculty were invited to a reception in May held to present the 2009 Dean’s Medals and to honor those who were at the forefront at the time of the move.
And now the action is back in the classroom. The fall quarter of the mini med school is full with 250 students. Registration for winter will begin Nov. 30. For more information: continuingstudies.stanford.edu. — Rosanne Spector



