SUMMER 2008 CONTENTS
Home
Good as Gold?
New drug approvals ebb; doubts over testing's gold standard grow
Q&A with Katie Couric
Standing up for cancer research
Breath of Hope
Lifeline or gamble? Sometimes a clinical trial is both
Just Another Lab Rat
The human subjects trade is booming, largely without oversight
Fixing Trial Tribulations
Solutions from Stanford
A Spoonful of Sugar Pills
Why nothing really is something, and in some ways is better than anything
Banding Together
Minds of all kinds join to hasten discoveries of new medical treatments


Breath of hope
Lifeline or gamble? Sometimes a clinical trial is both
By Ruthann Richter
Photos by Leslie Williamson
Darren Dent sits at his computer, breathing audibly, with the regularity of clockwork. He’s greedy for air.
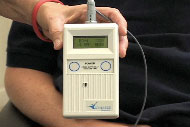
Dent, 42, suffers from ALS and can no longer take the act of breathing for granted: Respiratory failure is the most common cause of death among those with the neurodegenerative disease. So Dent’s hedging his bets on a clinical trial with a breathing device that he hopes will keep him alive. He is the first patient on the West Coast to be fitted with the apparatus, which aims to shore up the diaphragm — that thin, dome-shaped muscle beneath the lungs that helps us take in air. When switched on, the machine (about the size of a videotape) sends pulses of electricity to the diaphragm through fine wires implanted in Dent’s chest. Each pulse acts like an electronic push-up for the muscle, in theory helping to keep it functioning and fit.
For Dent, the trial is a lifeline — his only hope until something better comes along. He has lost his ability to move and can barely speak, communicating largely through a computer that’s adapted to respond to minute movements of his head. With a simple nod detected by a camera on top of the computer, he can choose a letter or word appearing on the computer screen.
“I have tremendous hope that I can buy enough time,” he says via the computer display. “Without that small glimmer of hope, I’d be moping around here all day.”
His Stanford physician, neurologist Yuen So, MD, feels a crying need for tools to help patients with amyotrophic lateral sclerosis, or ALS. But as a scientist, he is more circumspect in his approach to the trial.
“I think it’s OK to hope for the best. But it’s also important to be realistic,” says So, a professor of neurology and a principal investigator in the trial. “I think the clinician’s perspective is to help the patient deal with the disease, help them prepare for the worst and yet walk a fine line not to eliminate any last bit of hope. For any one individual, the disease course may be unpredictable.”
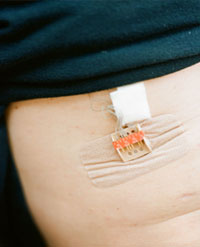
The stimulator plugs in on Darren Dent's
chest. The wires thread in through his skin and attach to his
diaphragm.
Once a successful businessman and athlete, Dent never imagined he’d become so steeped in the world of clinical trials, clinging to every prospect that comes along. A resident of Martinez, Calif., he used to play basketball and coached softball at his son’s school. In family photos he stands well above 6 feet alongside his wife and four children at the beach: a robust, broad-shouldered man with dark brown hair and greenish-brown eyes.
In 2005, Dent says he began to notice some disquieting symptoms. First his speech became slurred. Then came some weakness in his left hand and arm. Soon his legs started to give way. It wasn’t long before he was completely paralyzed and wheelchair-bound, able only to move his neck enough to signal computer commands.
It was the insidious decline of ALS, also known as Lou Gehrig’s disease. Dent was officially diagnosed in 2006; by March 2007, he was forced to give up his job as a business development manager for an Internet firm.
The disease mysteriously eats away at the nerves that control all voluntary muscles, including those involved in movement, speech, breathing and swallowing. It leaves the mind intact. The disease affects an estimated 30,000 Americans at any given time.
For Dent, it means that simple tasks such as eating and bathing have become laborious routines. Because swallowing is difficult, it can take as much as an hour for him to get his food down. “I used to live to eat,” he says, “and now I eat to live.” He cannot dress or go to the bathroom without help and spends hours in front of the computer, painstakingly crafting the e-mails that help him communicate with the outside world.
As Dent’s disease progressed, he and his caregivers
searched eagerly for treatment options, but did not come up with
much. He took part in one trial with a drug called ritonavir,
commonly used to treat HIV, and is waiting for those study
results. Then his doctors at the University of California-San
Francisco alerted him to a new clinical trial with a device
called a diaphragmatic pacemaker that had been used to help
patients with spinal cord injuries breathe. The late actor
Christopher Reeve was among those who had benefited from the
device. It gave Reeve several hours a day of blissful freedom
from his ventilator, which requires keeping a tube down the
throat, makes speech impossible and puts patients at the mercy
of an external device.
Parry Dent, Dent’s mother, a registered nurse and one of his primary caretakers, says when she learned of the pacemaker, “I thought, ‘This is pretty phenomenal stuff.’ I thought it was something significant that could help with ALS.”
The device was developed after two decades of research by scientists at Case Western Reserve University and University Hospitals of Cleveland. It is now manufactured by Synapse Biomedical Inc. in Oberlin, Ohio, which is sponsoring the latest multisite trial.
At Stanford, on Oct. 29, 2007, Dent received the experimental device in a procedure performed by John Morton, MD, an associate professor of surgery. Five other ALS patients at Stanford since have been implanted with the pacemaker, So says.
“The single most important thing that limits life in these patients is weakness of the muscles in the respiratory system,” So says. “That is where this trial comes in.”
He says the sites participating in the trial hope to enroll a total of 100 patients by the summer and have results ready for publication a year later. The company also is expected to submit results to the federal Food and Drug Administration, which requires sponsors to prove devices are safe and do what they purport to do — in this case, to effectively stimulate the diaphragm. The approval process is similar to that for drugs except that it requires no placebo control group for comparison.
So says a goal of the trial is to get patients on the device
before they become dependent on a ventilator, which often
signals that the end is near. Patients typically become
ventilator-dependent within three to four years of being
diagnosed. So cautions that the device is no panacea, as it
doesn’t treat the underlying disease, so patients will
continue to decline. But the hope is that with improved
breathing they will live longer and with better quality of
life.
Some patients have been reluctant to participate in the trial because they know their disease will continue to progress, regardless. “Some people say, ‘Why bother when you’re not improving the rest of me?’”
“With stimulation and conditioning of the diaphragm, we may be able to slow down the deterioration,” So says.
The device is installed in the operating room in a relatively simple procedure that takes about 90 minutes, says Morton, a co-investigator in Stanford’s portion of the trial. The procedure, performed through a laparoscope while the patient is under general anesthesia, involves creating four incisions, about a half-inch each, in the patient’s lower chest. Morton then methodically “maps” the diaphragm, searching for the strongest parts of the muscle, because the device works best when it’s connected to nerves and tissues that are still in relatively good shape. He manipulates a blunt instrument through one of the incisions and places it against the side of the muscle to test its strength, all the while viewing the results on a screen. A video of Dent’s surgery shows the pink muscle responding with a quick thump, a contraction — a good sign. Morton then pierces a tiny hole in that area of the diaphragm to place the wire and threads the end out of the body. A fifth wire grounds the device.
Now a small plastic case taped to Dent’s lower right side holds those five little wires. The stimulator can be programmed for different electrical currents and frequencies. His is set to make him breathe 14 times a minute. The breaths come in deliberate pulses, with an audible whoosh of exhaled air. He generally has someone turn the machine on for him about five times a day, half an hour at a time, and for a few hours while he sleeps. Patients limit their time on the device because it’s not known whether using it all the time does more harm than good, in effect exercising the muscle too much, So says.
Dent says the installation of the device was somewhat painful; it was three weeks before he felt fully recovered. He was also fitted with a feeding tube during the procedure, which might have prolonged the recovery process. Now he rarely feels pain from the pacemaker device — only once in a while, when he lies down.
“It’s added one more mundane task to a long list of things that we have to do every day,” he says. “But it is worth it because I do feel as if I have more energy, stamina and can breathe easier.”
Parry Dent, who is with him most days, says the benefits are noticeable. Her son is able to get more words out — an unexpected bonus — and tires less easily. He’s more involved in family activities, attending his daughter’s softball games and going on more frequent outings. In the spring, he also went on a whirlwind, nine-day trip with his parents to the Grand Canyon, Sedona and Las Vegas, riding in his specially equipped van. It’s something he probably couldn’t have done before the device was installed, he says.
The device is experimental and has risks, doctors caution. As with any surgical procedure, infection and hemorrhage may occur, though there have been few complications thus far, So says. He says some patients have been reluctant to participate in the trial because they know their disease will continue to progress, regardless.
“Some people say, ‘Why bother when you’re not improving the rest of me?’”
In Dent’s wilder imaginings, he sees the technology being applied to other parts of the body, essentially freeing him from his paralysis — though no physician has proposed this as a possibility.
“Maybe they could apply similar technology to legs, arms, throat,” he says, though he concedes, “I don’t know how practical that is.”
Despite the risks, Dent says he never hesitated for a moment to sign up. “The disease has so few options for treatment,” he says. “What is there to lose?”

Dent's Device
A visit with the Dent family and a look at Darren Dent's device in clinical trial.