Mission: Translational
[The expression "translational medicine" sums up Stanford University Medical Center's top priority: translating the insights of students, clinicians and scientists into practical advances that enhance and prolong life.]
 |
|
Michele Calos, PhD, has solved one of gene therapy’s major problems. |
|
Michele Calos has developed a technique that guides therapeutic genes to safe places on the chromosome
By Amy Adams
Photographs by Meredith Heuer
Illustration by Ian Worpole
Until recently, gene therapy faced a damned-if-you-do, damned-if-you-don’t
dilemma. In the safest form of gene therapy, a gene inserted into the
cell survives briefly and makes a necessary protein, but the gene eventually
breaks down and its therapeutic effect fizzles away. A longer-lasting
treatment involves wedging the gene into a chromosome, where its benefits — or
detriments — last indefinitely. The long-lasting effects would be
a therapeutic boon except for the collateral damage inflicted by the
gene as it inserts into the chromosome.
This problem came to a head in the fall of 2002, when the first successful
gene therapy trial turned sour. The trial, based at the Necker Hospital
for Sick Children in Paris, was for severe combined immunodeficiency,
or SCID — otherwise known as the “bubble boy” disease — in
which some immune cells don’t mature, leaving a child susceptible
to any infection that comes along. A gene inserted into cells of the
bone marrow stimulated new immune cells to form and cured nine of the
11 children in the trial. But then two of those children developed leukemia,
probably because when the therapeutic gene elbowed its way into a chromosome
it activated a neighboring leukemia-causing gene. That trial, and 27
other gene therapy trials for otherwise incurable diseases, abruptly
ended.
It was in this environment that Michele Calos, PhD, published papers
in Nature Medicine and Nature Biotechnology describing
a new gene therapy technique that avoids the pitfalls of older approaches.
Unlike therapies in which the gene survives only briefly, her approach
allows the gene to enter the chromosome, where its effect is long-lived.
And with Calos’ technique, the gene enters the chromosome only at
known genetic addresses, which so far seem nowhere near problematic genes.
“Ever since we released those papers I’ve had a continuous
flurry of e-mail,” says Calos, associate professor of genetics at
the School of Medicine. “The leukemia patients really put into focus
a basic flaw with current gene therapy techniques.”
The “aha” moment
The key to gene therapy is inserting a healthy copy of a gene into the
cells of a sick person, then hoping that gene will make a normal protein
to cure the disease. To pull off this feat, most researchers take advantage
of a retrovirus, a type of virus that inserts its genes into human DNA.
In gene therapy, researchers replace the disease-causing viral DNA with
their therapeutic gene, then let the virus do the hard part of integrating
the gene into a chromosome.
The problem is that the retrovirus is not very discriminating. Sometimes
it lands in a harmless location, but other times it damages its genetic
neighbors in the process of moving in. It’s this recklessness that
led to problems in the SCID trial.
While attempting to devise a better gene therapy technique, Calos noticed
a research article that seemed interesting enough to take home for a
careful read. “I read the paper that night and I literally put a
graduate student on that project the next day. I realized immediately
that it was exactly what we needed,” Calos says.
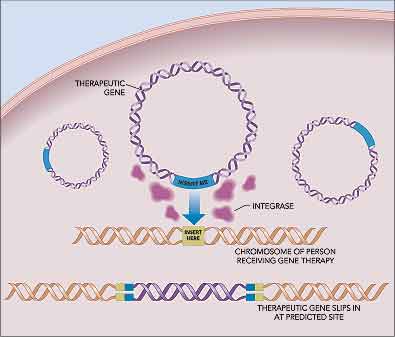 |
|
Goodbye guesswork: By guiding new genes to known locations, integrase turns gene therapy into more of an exact science. |
|
The paper described a protein called an integrase that inserts genes
into a precise location marked by a genetic “insert here” sequence
in the bacterial chromosome. It turns out that humans also have several
gene regions mimicking that sequence scattered throughout the genome.
Calos thought that if the integrase could recognize those pseudo-integration
sites in humans, she could create the first gene therapy technique that
guides the insertion of genes into human chromosomes.
“It’s one of those serendipitous things. Once we knew about
the integrase everything just fell into place and no huge roadblocks
came up,” Calos says.
Her technique has only two components — a therapeutic gene flanked
by an “insert me” sequence and the integrase. The integrase
inserts the therapeutic gene into one of several sites in the human genome;
then the integrase breaks down over the next few days leaving only the
inserted gene as evidence of its genetic tinkering.
Put theory into practice
Calos initially tested her approach using a gene that makes factor IX — a
protein missing in the blood of people with one form of hemophilia. She
and Mark Kay, MD, PhD, a Stanford genetics professor who specializes
in gene therapy techniques, injected mice with a piece of DNA containing
the factor IX gene. Within a week, mice that received this injection
had 12 times more factor IX in the blood than their littermates that
received the injection without the integrase. That’s enough factor
IX to treat a person with hemophilia.
In addition to working on hemophilia, Calos has responded to the worldwide
interest in her approach by joining forces with researchers studying
more than a dozen different diseases. “A lot of people working with
retrovirus need a solution, so they are trying to switch over to integrase,” Calos
says. The collaborations haven’t left Calos pining for the quiet
days of yestermonth. “This is so much more fun with more people
involved,” she says.
Teaming up
One of her collaborators is Paul Khavari, MD, PhD, who did the initial
work with human skin cells. She and Khavari were working with skin cells
from patients with a disabling disease called recessive dystrophic epidermolysis
bullosa, in which the outer layer of skin separates too easily from the
underlying layers. Children with the disease have severe blistering,
scarring, infections, and they often die young.
She and Khavari, a Stanford professor of dermatology, injected a healthy
copy of the gene that’s mutated in epidermolysis bullosa into skin
cells taken from children with the disease. They then allowed those cells
to multiply in a lab dish and placed them on the skin of a mouse. Once
in place, those cells formed normal skin and made a normal copy of the
protein that located to the appropriate place in the skin cells.
Another Stanford collaborator of Calos’ is Thomas Rando, MD, PhD,
who works on muscular dystrophy — a disease that cries out for gene
therapy, according to Rando. “The only treatment for any form of
muscular dystrophy is very unsatisfactory,” he says. Because the
most common form of the disease is caused by a mutation in a single gene,
it is a perfect candidate for gene therapy.
The integrase gene therapy offers an added bonus for treating muscular
dystrophy. The gene that’s most commonly mutated in this disease,
called dystrophin, is too large to fit within the narrow confines of
most viruses. Because Calos’ technique does away with stuffing genes
into viral containers, researchers can inject the genetic behemoth into
cells with a reasonable hope of it integrating into human chromosomes.
This same advantage holds true for treating cystic fibrosis and epidermolysis
bullosa, both of which are caused by mutations in large genes.
The problem for gene therapy in muscular dystrophy is one of delivery,
says Rando, associate professor of neurology and neurological sciences
and director of the muscular dystrophy clinic. “Muscle is distributed
throughout the whole body. You have to get the gene to muscles ranging
from the leg to the heart to the diaphragm. It’s a major delivery
problem.”
Research with mice shows that a gene injected directly into muscle can
integrate into local cells, but a person with muscular dystrophy needs
more than a few square inches of treated muscle. One possible approach,
Rando says, is to inject dystrophin genes and integrase proteins into
an artery where the blood can then shuttle them throughout the body.
Rando and his colleagues are working on ways to keep the DNA intact and
able to enter muscle cells after it is injected into the blood.
From mice to men
Problems with gene delivery crop up any time a gene has to work its
way into a particular cell type. That’s one reason Calos expects
gene therapy for hemophilia to be her first project to make it to human
trials. In this blood disease it doesn’t matter what tissue contains
the therapeutic gene, as long as the protein winds its way into the blood
supply. Inserting a gene into either muscle or skin cells, both of which
Calos knows are compatible with her technique, has the potential to release
therapeutic proteins into the blood.
Though Calos’ current work is in animals or human cells, her goal
is to see her brainchild used to treat human patients. “You are
seeing the beginning of a new era in gene therapy for a bunch of diseases,” Calos
says. “My goal is to see this really used in medicine.”
Getting a therapy to human trials takes money, and money usually comes from pharmaceutical companies or private investors. So Calos and two colleagues have started a company to move her technique into the clinic. They named the company Poetic Genetics because, says Calos, like words in a poem the magic of gene therapy is to create a lasting impression. Poetic Genetics has begun the task of finding potential funders who aren’t scared off by either the limping economy or fears about gene therapy. A few investors meet both of those criteria, leaving Calos hopeful that through Poetic Genetics her technique will one day make a lasting impression on genetic diseases.
Comments? Contact Stanford Medicine at

