Consider the basic building blocks
of most organisms – molecules, genes, cells. And tubes. Though tubes
don’t spring to mind as readily as the others, they are one of the
most fundamental structures found in internal organs. The lungs, kidneys,
vascular system, liver, pancreas and mammary glands are all made of branched
tubular structures that transport gases and liquids.
But relatively little is known about how these biological tubes form.
For instance, how do cells make tubes, and what regulates the size and
shape of the tubes? And how do they branch into complex patterns that
ensure an adequate flow of gases and liquids to various parts of the body?
The answers to these questions have profound implications for understanding
both their normal formation during development and what happens when development
goes awry, resulting in disease or the growth of a tumor in these structures.
And with precise knowledge of how such diseases and tumors form, scientists
could design molecular cures to pinpoint and repair the trouble spots.
But first they need a solid understanding of the tube formation process.
Enter Mark Krasnow, MD, PhD, a Stanford professor of biochemistry and
an investigator for the Howard Hughes Medical Institute.
For the past 10 years, Krasnow and a tenacious team of researchers have
studied the tracheal system of the fruit fly, properly known as Drosophila.
Their efforts have resulted in a cellular-level understanding of how the
simple tubular structure forms. The team plans to spend the next few years
refining that knowledge while also using it to guide similar research
into the formation of a vastly more complex system — the mouse lung.
To do that, the team is using the latest technologies and “old-school”
science — patiently researching literature, following leads that
sometimes turn into blind alleys and developing simple descriptions of
the processes.
“What we’re doing is rather unusual. There are people who
are studying mouse lung development and there are people who are studying
Drosophila respiratory system development, but as far as I know
we’re the only ones trying to do both,” Krasnow says.
And already the unique approach has provided some surprising results.
The team has discovered that the genetic process controlling the sprouting
of branches in Drosophila’s simple tracheal system is remarkably
similar to that of the highly complex mammalian lung.
“The findings are surprising because, although the respiratory system
in Drosophila and the lung have similar physiological functions,
no evolutionary biologist had thought these structures would be under
similar genetic control,” Krasnow says. “It raises the possibility
that the tracheal system in Drosophila and the lungs in mammals
might not only be functionally similar but they might have some evolutionary
relationship as well.”
Krasnow succumbed to the siren song of Drosophila more than 15
years ago. He had come to Stanford in the 1980s as a postdoctoral fellow
working in the lab of David Hogness, the emeritus Rudy J. and Daphne Donohue
Munzer Professor in the School of Medicine, at a time when Hogness and
others were pioneering techniques that enabled them to understand how
genes controlled the developmental processes of the fruit fly.
“The biggest successes during that time came with understanding
some of the very first events during development — the events that
happen right after fertilization that establish the major body axes and
that make the head different from the tail and the back different from
the front,” Krasnow says. “The results were so powerful in
providing an initial molecular and genetic understanding of those developmental
processes that it encouraged people like me to say, ‘If this can
be done with the early developmental events, why not look at some of the
later developmental events, like the ones that occur during organ formation?’”
Using techniques that had shed light on the early developmental processes,
Krasnow and others made great leaps in understanding how Drosophila’s
organs form. Like most researchers, Krasnow chose to concentrate on a
single organ. He selected the tracheal system — the intricate, branched
structure of threadlike tubes that carry oxygen to the cells of the fruit
fly’s body.
“Branched tubular structures are one of the most fundamental structures
of organ design,” Krasnow says. Drosophila’s tracheal
system made an excellent model for studying the formation of branched
structures because of its simplicity — each tube is made of a single
layer of cells without any surrounding support structure. “You can’t
make a simpler cellular tube,” Krasnow notes. And Drosophila’s
tracheal system consists of a mere 10,000 branches, compared with the
millions of branches in the mammalian lung.

Mark Krasnow
“There are 80 cells that make up the couple hundred branches that
form in each segment, so you can get down to a cellular level of understanding
this problem. In fact, we’ve named every cell in the larval tracheal
system,” he says.
Krasnow’s Drosophila team includes postdoctoral fellows Amin
Ghabrial and Mark Metzstein, graduate students Farhad Imam and Stephanie
Toering, and until recently, former postdoc Eric Johnson, who in September
moved to the University of Oregon as an assistant professor of biology.
The researchers use genetic, cellular and molecular methods to identify
and characterize the genes involved in the development process.
Johnson, who works primarily on understanding the branch patterning of
the tracheal system, says he was initially drawn to the work for aesthetic
reasons. “I thought the Drosophila tracheal system was very
beautiful,” he says. “And then I figured that there must be
some really interesting biology behind it.”
The team discovered that the cells in the branch segments form a sac from
which small groups of cells begin to migrate in different directions as
they form the primary branches of the tracheal system. The primary branches
sprout secondary branches and then the fine terminal branches.
The team has documented how the branches sprout and what the cells are
doing during the branching events. “This gives us a cellular-resolution
view of these complex organ formations,” Krasnow says. Their work
has involved identifying, cloning and characterizing each gene to determine
how it contributes to the overall process of sprouting branches and forming
tubes.
Krasnow’s team named the genes — bestowing such monikers as
“branchless,” “sprouty,” “pruned,”
“stumps” and “trimmed” — and worked to characterize
the molecules they encode. As the scientists studied the branchless gene,
they discovered it produced a fibroblast growth factor similar to one
produced in mammals. Fibroblast growth factors, or FGFs, are powerful
substances implicated in the development of branched structures, such
as blood vessel networks and lungs.
“We began to understand how the Drosophila branchless FGF
controlled branching,” Krasnow recalls. “It was very exciting
because David Sutherland [a graduate student at that time] found that
the gene turns on just before branching begins, but it’s not expressed
in the tracheal cells themselves. Rather, it’s expressed in clusters
of cells that surround the tracheal system at every position where a new
branch will soon sprout. The tracheal cells sprout new branches by growing
out toward these FGF signals that are basically a chemo-attractant —
a come-hither signal — that tells the branches where they should
grow.”
Further research indicated that the influence of the branchless FGF extends
beyond the formation of the primary tracheal branches. As the primary
branches approach the FGF signal centers, genes involved in the secondary
branching event are activated. “And then we found more recently
that the gene turns back on again a couple of hours later, now in a completely
different pattern, and controls the sprouting of the very fine terminal
branches,” Krasnow says. “That was a surprise because we didn’t
expect to find a signal that would control so many important aspects of
the complex branching structure.”
The first two stages of branching — the primary and secondary branching
— are controlled genetically so that the process always turns on
in the same places. “It’s highly reproducible, highly stereotyped;
it’s part of the hard-wired developmental program,” Krasnow
says. But a key discovery by Johnson and postdoctoral fellow Jill Jarecki
showed that when the terminal branches start to form at the end of embryogenesis,
the process switches to physiological control, meaning that the oxygen
needs of individual cells in the body dictate where the terminal branches
form.
“This makes a lot of sense because the initial hard-wired program
gets the main branches out near the target tissues,” Krasnow says.
“But to get the very fine branches out to the cells in the tissues,
the process is controlled by oxygen. Any cell that starts to become starved
for oxygen — either because it has used up its available supply
or because it happens to be in a region that hasn’t received any
tracheal branches — somehow knows that it’s in an oxygen crisis
and responds by turning up expression of this FGF gene.
“So you have this intricate feedback system, which ensures that
the branches, although they look very random in their distribution, are
actually very precisely guided to the individual cells in each tissue
that need oxygen the most.”
It didn’t take long for mammalian parallels to crop up. Within the
last two years, other researchers exploring mammalian lung development
reported that sprouting of new bronchial branches is substantially controlled
by an FGF and an FGF receptor expressed in the cells that line the pulmonary
passages. “In addition, we discovered a gene called sprouty in Drosophila
that was an antagonist — an inhibitor — of branching,”
Krasnow says. “It was a new gene, but we soon found mammalian homologs
for sprouty in mice and also in humans.”
By the end of the 1990s, Krasnow knew his research team had made considerable
advances in understanding the formation process of the tracheal system
in Drosophila. “We’ve made enough progress that at
least the questions we have to answer are now clear. That means that the
project is maturing,” he says.
But as the researchers sought to compare mammalian lung development with
the findings about the Drosophila tracheal system, they realized
that much regarding mammalian lung development was murky. For one thing,
most of the initial descriptions of lung development were at least 40
years old and were written before the availability of the powerful microscopes
and reagents commonly used in today’s laboratories.
And yet, few scientists seemed interested in revisiting those descriptions.
“In today’s science, if you were to write a grant to the NIH
and say you want to describe the gross branching pattern of the mouse
lung, the reviewers would say, ‘Wasn’t that done a half-century
ago? I think you should find another source of funds,’” Krasnow
says.
He decided to assemble a second team in his lab to launch a parallel investigation
into the development of the mouse lung. Current team members include MD/PhD
student Ross Metzger and postdoctoral fellow Hernan Espinoza. For the
past few years, Metzger has been mapping out the sprouting process in
the mouse lung and looking at how the blood vessels, smooth muscle cells
and cartilage develop in concert with the bronchial tree. Metzger characterizes
his work as “old-school science with new techniques.”
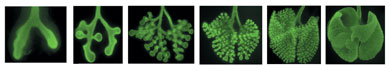
Mouse lungs: Day 11. Embryonic mouse lungs.
Primary bronchi have formed. Day 12: Primary brnochi elongate and additional
bronchi bud. Day 13: The newly formed bronchi are smaller than those formedearlier.
Day 14: The lungs' five lobes take shape - one in the left lung and four
in the right. Day 15: At this stage, the lungs measure about 1/4 inch
across.
“What I’ve done is not glamorous,” Metzger says with
a laugh. But he notes that he was able to pioneer a method that allows
him to stain and microscopically examine the entire mouse lung. Previously,
researchers were only able to stain and examine slices of the developing
lung. The beauty of Metzger’s technique is that it allows researchers
to see the formation of the airways in the developing lung in three dimensions.
“The first time I saw this gorgeous, elaborate network of mesh,
it was unbelievable,” Metzger says.
Additionally, Espinoza has been examining the molecules and their expression
patterns during these developmental branching events. Espinoza has also
been leading the effort in the first large-scale gene expression screen
in the lung, which will provide an overall look at the major gene expression
patterns during mouse lung development. “Because the sequences of
all those genes are known, when we find genes that are expressed in an
intriguing pattern we can often make pretty reasonable predictions as
to how they may be functioning in lung development,” Krasnow says.
“The genomics is going to lead the genetics in mouse lung development,
rather than the way it worked in Drosophila where the genetics
has led the genomics.”
Though it has been slow going during the past few years for the lab’s
mouse lung research, Krasnow believes it’s laying an essential foundation.
“We’ve taken an approach that others in the field of lung
development hadn’t seen as critical,” he says. “This
type of foundation is really necessary to get to the kind of genetic and
molecular detail achieved in the Drosophila tracheal system. I
think our approach is beginning to be appreciated now that we’ve
started talking about it at meetings. It’s opening up a new understanding,
and I think it heralds a renaissance in understanding lung development.
“I hope that these careful groundwork studies for an organ like
the lung will encourage other laboratories around the world to spend a
similar amount of time in setting up the framework for understanding the
development of other important organs.”
The information from the gene expression screen will give Krasnow’s
team both genetic and developmental overviews of the mouse lung development
process. If the research follows the pattern established by the Drosophila
work, the overviews will then guide the team in relating specific genes
to discrete developmental events and eventually to identifying all of
the genes involved in lung development. “It’s fun to do it
in stages because you get a lot of intellectual gratification from providing
the initial outline,” Krasnow says. “Then in the second phase
there’s more intellectual gratification in seeing the genetic program
come into view.”
And having the Drosophila research serve as a conceptual guide
should help speed up the progress in the mouse lung work in the years
ahead. “We have to figure out ways to make the genetic and molecular
approaches move faster than they traditionally have for the mouse lung
research, but that’s one of the challenges we think can be partially
overcome by the genome projects that are going on,” Krasnow says.
“When we started with Drosophila, just finding individual
genes was a big deal. But now with the mouse and with humans, you’re
starting with literally the whole catalog of genes. We just have to figure
out the ones that are relevant for lung development.”
Though the challenges of the mouse lung project are great, Krasnow points
out that the goal of the research is the same as for the Drosophila
project: To understand the complete developmental program of a key organ.
And once researchers have obtained a genetic understanding of how lungs
form in mice – and, eventually, in humans – they can begin
to understand how the normal developmental program can go wrong, resulting
in lung cancer and other diseases. “It will suggest ways of treating
those diseases,” Krasnow says.
“Researchers will begin to make reasonable predictions as to how
to fix them in a way that’s never before been possible. Ultimately,
it might even be possible to trigger the lung development program in an
adult so that if, for instance, a portion of a lung is resected because
of a tumor, we could reactivate the development program in the residual
lung to generate a new lobe and restore functionality.”
Although that scenario may be decades away, Krasnow has seen enough advances
during his career to believe that it’s within mankind’s grasp.
“A few years ago, I wouldn’t have thought it was possible
to do a project like mouse lung development or to even think about the
possibility of regenerating a lung. It was too complicated, and finding
the genes and understanding the process seemed like it was so far in the
future that it wasn’t even worth spending time on,” he says.
“But I’ve been so encouraged by how much progress the lab
has made in the 10 years of studying the Drosophila tracheal system
that, with all the new technologies, I think we’re really in reach
of knowing the complete developmental program for the Drosophila
tracheal system.”
Perhaps advances in understanding mammalian organs are also close at hand.
Says Krasnow: “We’re also at least in the position to be thinking
that it’s possible — in our lifetime — to gain a similarly
high level of understanding of lung development, blood vessel development
and the development of other organs.”
SM |
![]() M
E D I C I N E
M
E D I C I N E